eCTD Outsourcing
eCTD Outsourcing offers a cost-effective and flexible way to manage global regulatory submission.
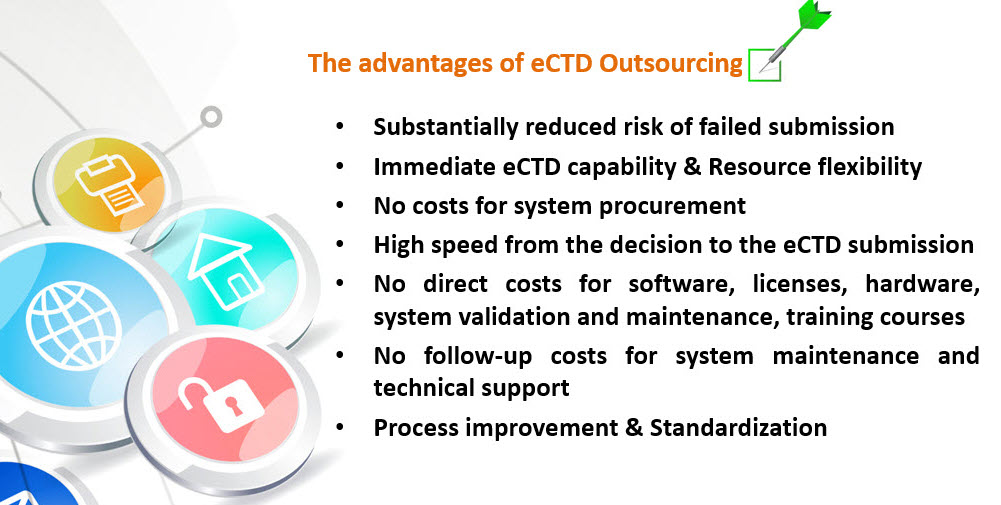
RegOrbit support you in an effective way to keep your company and your internal resources to focused on your core competency of drug development and approval while we efficiently build and submit your eCTD submissions to global health authorities.
Contact Us at info@regorbit.com or +43 68110531332