
FDA Data Standardization initiative – REMS in SPL mandatory submission from 28th December 2022
FDA has finally published a draft guidance “Providing Regulatory Submissions in Electronic Format –Content of the Risk Evaluation and Mitigation Strategies (REMS) information Using Structured Product Labeling (SPL)” on 23rd December 2020 under FD&C 745A(a) for review and comments. This new REMS electronic submission in SPL requirements officially take effect 2 years from the publishing of this guidance.
What is REMS
A Risk Evaluation and Mitigation Strategy (REMS) is a drug safety program that US FDA can require for certain medications with serious safety concerns to help ensure the benefits of the medication outweigh its risks. REMS include a risk mitigation goal and are comprised of information communicated to and/or required activities to be undertaken by one or more participants (e.g., health care providers, pharmacists, patients) who prescribe, dispense, or take the medication. Together, the goal, communications and/or activities make up the safety strategy.
Need of Standardized REMS
- Currently REMS are described in a variety of ways, and REMS requirements are often unclear to stakeholders
- The format of REMS documents/materials varies
- REMS lack consistent terminology – Similar concepts often have different names – Different concepts may have the same name
- REMS are often described using regulatory terms like “ETASU”, “Communication Plan” and “Element A-F” and it does not provide clarity about how REMS programs work
Why REMS need to be submitted in SPL
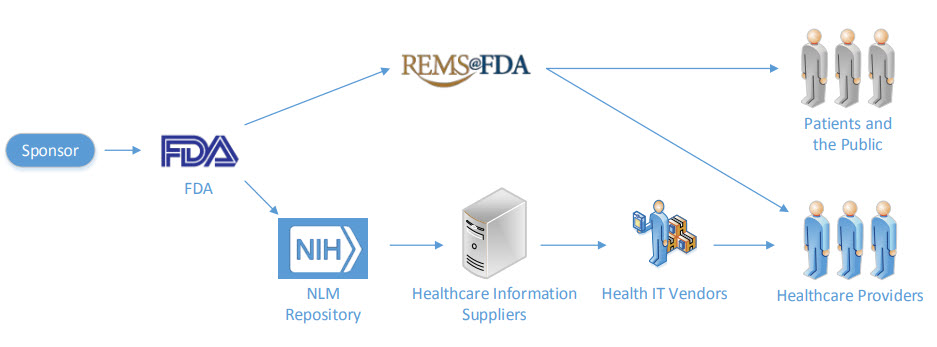
Since REMS materials and requirements are difficult to locate and the specific activities and Requirements of various stakeholders (e.g., prescriber, pharmacist) are not always clearly summarized and needs to be find a better or alternative ways to integrate REMS materials and procedures into an existing health information systems and health care delivery processes. Incorporating and submitting standardized REMS data in an existing standard format like SPL can help to easily integrate REMS into the healthcare systems and ensures stakeholder awareness of and compliance.
Advantages of submitting REMS in SPL
- REMS Summaries will be transformed into standardized data elements
- Makes REMS information easier to understand
- Makes REMS information more accessible
- Helps integrate REMS into the health care process
- Approved REMS will be available on DailyMed
Sponsors could submit their REMS in SPL format as soon as your REMS SPL file is approved, it will be made available on DailyMed. The REMS information in SPL is shared across the healthcare systems and REMS data is expected to be transmitted from the sponsor to patients, healthcare providers, and to the public.
Refer below mentioned link to find FDA draft guidance for your review
Send your question or feedback if any to info@regorbit.com
